|
PLUTO
|
|
PLUTO
|
Compute the mean molecular weight. More...
#include "pluto.h"
Go to the source code of this file.
Functions | |
| double | MeanMolecularWeight (double *v) |
Compute the mean molecular weight.
Compute and return the mean molecular weight as a function of the gas composition under non-equilibrium conditions. The mean molecular weight is usually needed to compute the temperature or mass density from the particle number density:
![\[ T = \frac{p}{n_{\rm tot}k_B} = \frac{p}{\rho}\frac{m_u\mu}{k_B} \,,\qquad \rho = \mu m_u n_{\rm tot} \]](form_56.png)
where 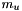 is the atomic mass unit while
is the atomic mass unit while 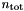 is the number density of all particles. The Mean molecular weight is defined as the average mass of a particle of gas in terms of the atomic mass unit and it is expressed by the weighted sum of the mass of particles in atomic mass unit divided by total number of particles (see book by Ryan & Norton [Eq. 1.7])
is the number density of all particles. The Mean molecular weight is defined as the average mass of a particle of gas in terms of the atomic mass unit and it is expressed by the weighted sum of the mass of particles in atomic mass unit divided by total number of particles (see book by Ryan & Norton [Eq. 1.7])
![\[ \mu = \frac{\DS\sum_k N_k \frac{m_k}{m_u}}{\DS\sum_k N_k} \]](form_59.png)
where
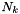 is the number of particles in the gas of element k and it can be related to mass fraction
is the number of particles in the gas of element k and it can be related to mass fraction 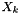 as
as 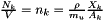 , where
, where 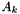 is the atomic mass number.
is the atomic mass number.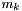 is the mass of each particle of element k.
is the mass of each particle of element k.H2_COOL: to compute 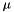 we proceed as follows:
we proceed as follows:
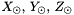 we derive
we derive
![\[ \frac{N_{He}}{N_H} = \frac{Y_{\odot}}{A_{He}}\frac{A_H}{X_\odot} ,\,\qquad \frac{N_Z}{N_H} = \frac{Z_{\odot}}{A_{Z}}\frac{A_H}{X_\odot} \]](form_67.png)
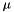 is given by
is given by
![\[ \sum_k N_k \frac{m_k}{m_u} = N_H \frac{m_H}{m_u} + N_{He}\frac{m_{He}}{m_u} + N_Z \frac{m_Z}{m_u} \]](form_68.png)
![\[ \sum_k N_k = N_{HI} + N_{HII} + N_{H2} + N_e + N_{He} + N_Z + \frac{A_ZN_Z}{2} \]](form_69.png)
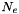 electrons corresponding to
electrons corresponding to 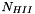 protons due to ionization of hydrogen and
protons due to ionization of hydrogen and 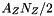 number of electrons due to metals. Note that now the electrons contribute to the total number of particles and cannot be neglected.
number of electrons due to metals. Note that now the electrons contribute to the total number of particles and cannot be neglected.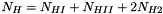 as the sum of number of atomic hydrogen (HI), ionized hydrogen (HII, or protons) and twice the number of molecular hydrogen (H2) and the corresponding number fractions:
as the sum of number of atomic hydrogen (HI), ionized hydrogen (HII, or protons) and twice the number of molecular hydrogen (H2) and the corresponding number fractions:
![\[ f_{HI} = \frac{N_{HI}}{N_H},\quad f_{HII} = \frac{N_{HII}}{N_H},\quad f_{H2} = \frac{N_{H2}}{N_H},\quad \]](form_74.png)
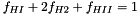 .
.Putting it all together:
![\[ \mu = \frac{A_H + A_{He}f_{He} + A_Zf_Z} {f_{HI} + f_{H2} + 2f_{HII} + f_{He} + f_Z + A_Z f_Z/2} \]](form_76.png)
where
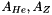 are atomic mass numbers of helium and metals respectively.
are atomic mass numbers of helium and metals respectively.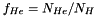 is the fixed number fraction of helium with respect to hydrogen;
is the fixed number fraction of helium with respect to hydrogen;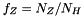 is the fixed number fraction of metals with respect to hydrogen.
is the fixed number fraction of metals with respect to hydrogen.MINEq: please see Eq. [12] of Tesileanu (2008)
SNEq: the derivation is similar to H2_COOL with 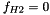 yielding
yielding
![\[ \mu = \frac{A_H + A_{He}f_{He} + A_Zf_Z} {2 - f_{HI} + f_{He} + 2f_Z} \]](form_81.png)
where one electron from metals is assumed.
No Chemistry: in case where chemical reaction are not incuded, the mean molecular weight is computed from the mass fractions assuming a fully ionized gas:
![\[ \mu = \frac{A_H + A_{He}f_{He} + A_Zf_Z}{2 + f_{He} + f_Z(1 + A_Z/2)} \]](form_82.png)
References
Definition in file mean_mol_weight.c.
| double MeanMolecularWeight | ( | double * | v | ) |
Return the mean molecular weight.
| [in] | v | array of primitive variables (including ions) |
Definition at line 120 of file mean_mol_weight.c.
